The European Parliament’s Environment Committee is the most active Committee scrutinizing the Commission’s output of secondary legislation.
Since the 9th legislature sat for the first time on 10 July 2019, the Environment Committee has seen 70 challenges tabled.
Updated 8 July 2022
A review of the challenges of the Environment Committee
10 July 2019 –
Objection to the extension of the approval periods of the active substances –flumioxazine and others
Implementing Act
Rapporteur: Anja Hazekamp (GUE/NGL)
Debate in Committee: 25 September 2019
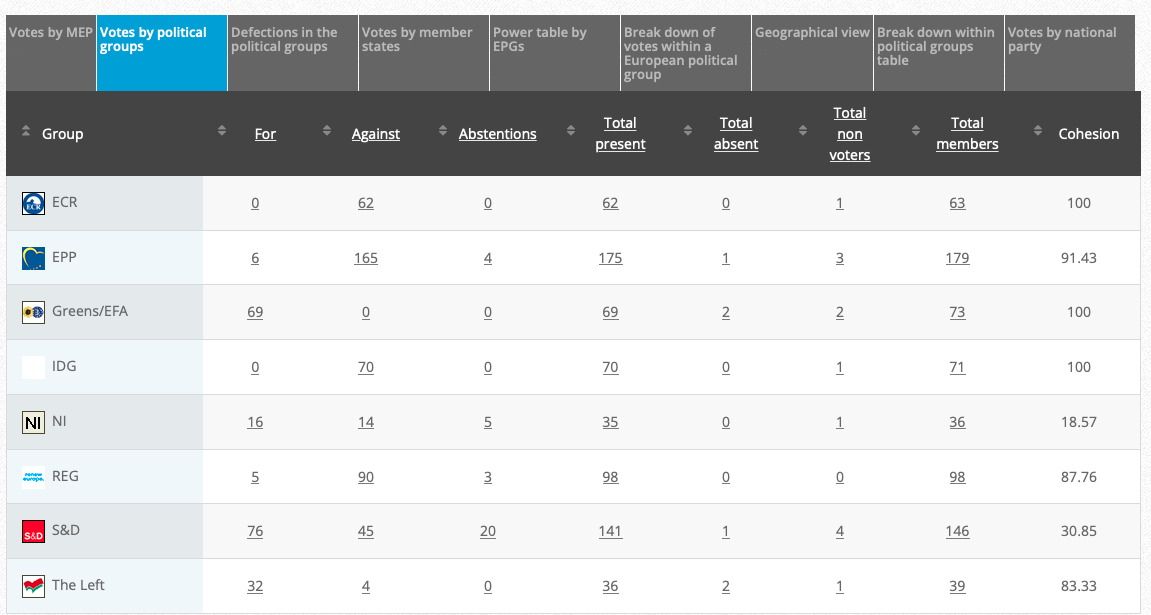
Adopted: For 47; against: 22; abstention(s): 1. Link
Vote in Plenary: 10 October 2019
Vote: For 415, Against 252, Abstentions 2o
Votewatch link
Commission’s follow up: SP(2019)669-2019:2825
2.
Objection to the extension of the approval periods of active substances
Implementing Act
Rapporteur: Anja Hazekamp (GUE/NGL)
Debate in Committee: 25 September 2019
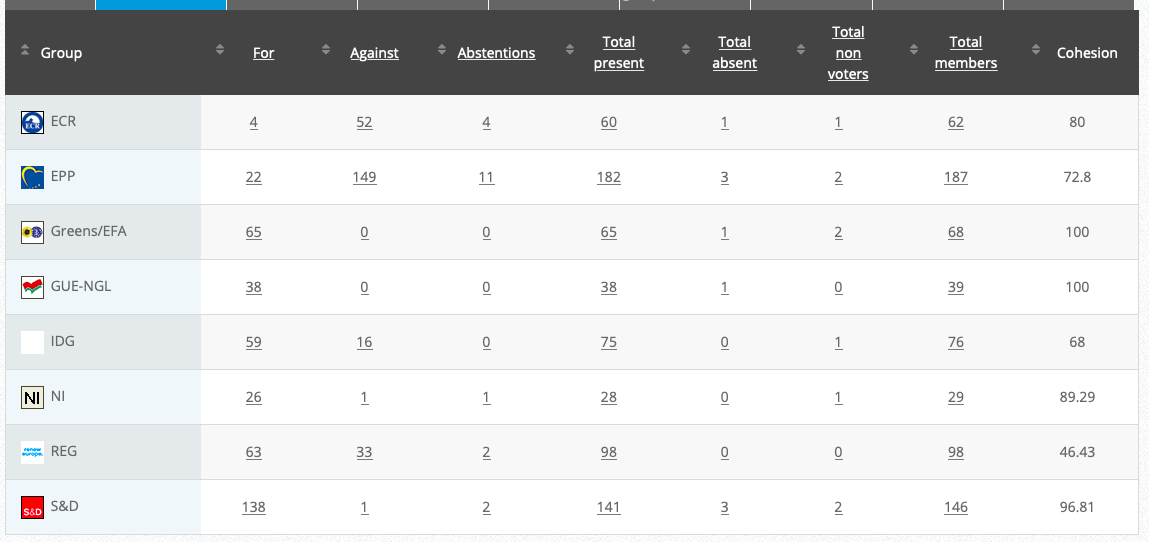
Adopted: For 49; Against: 20; abstention(s): 1. Link
Vote in Plenary: 10 October 2019
Vote: For 402, Against 222, Abstentions 39
Votwwatch link
Commission’s follow up:
3
Issue: Objection to GMO Maize
Implementing Act
Co-Rapporteurs: Sirpa Pietikäinen (PPE) Günther Sidl (S&D) Nicolae Ştefănuță (Renew) Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL) Eleonora Evi (NI)
Debate in Committee: 25 September 2019
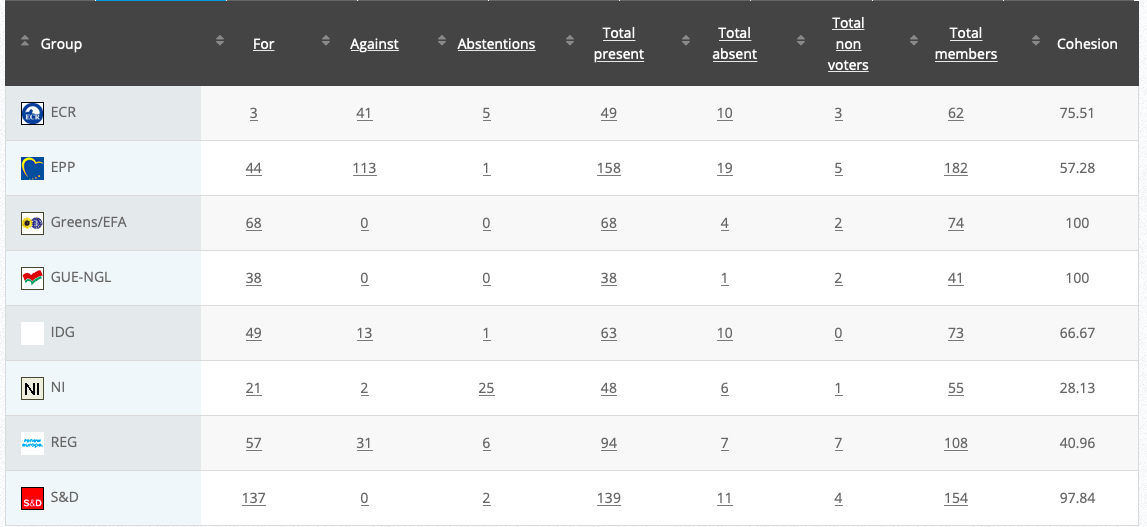
Vote: For 51, Against 15, Abstentions 5 Link
Vote in Plenary: 10 October 2019
Vote: For 436, Against 208, Abstentions 16
Votewatch link
Commission’s follow up:
4.
Implementing Act
Co-Rapporteurs: Sirpa Pietikäinen (PPE), Günther Sidl (S&D), Nicolae Ştefănuță (Renew), Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI)
Debate in Committee: 25 September 2019
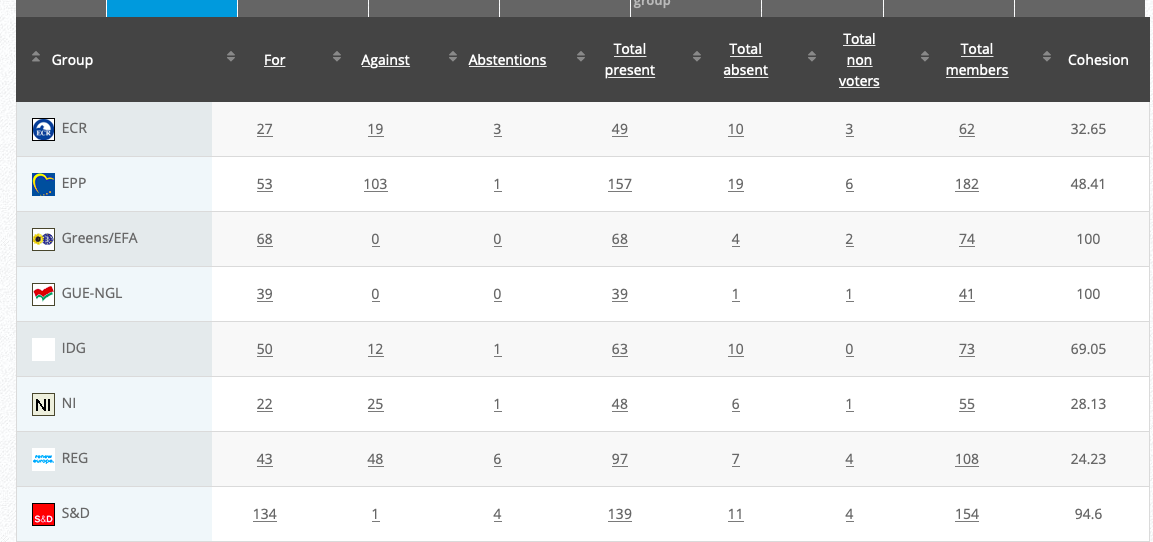
Vote: For 50, Against 14, Abstentions 7 Link
Vote in Plenary: 10 October 2019
Vote: For 426, Against 208, Abstentions 20
Votewatch link
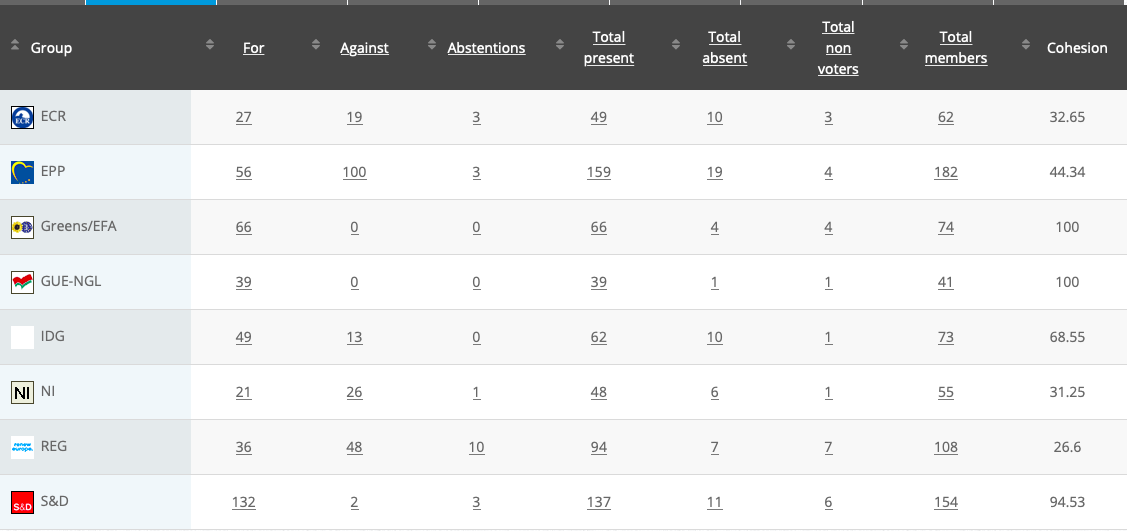
See above debate
Commission’s follow up:
5.
Implementing Act
Co-Rapporteurs: Sirpa Pietikäinen (PPE), Günther Sidl (S&D), Nicolae Ştefănuță (Renew), Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI)
Debate in Committee: 25 September 2019
Vote: For 50, Against 16 Abstentions 4 Link
Vote in Plenary: 10 October 2019
Vote: For 435, Against 207, Abstentions 18
Votewatch link
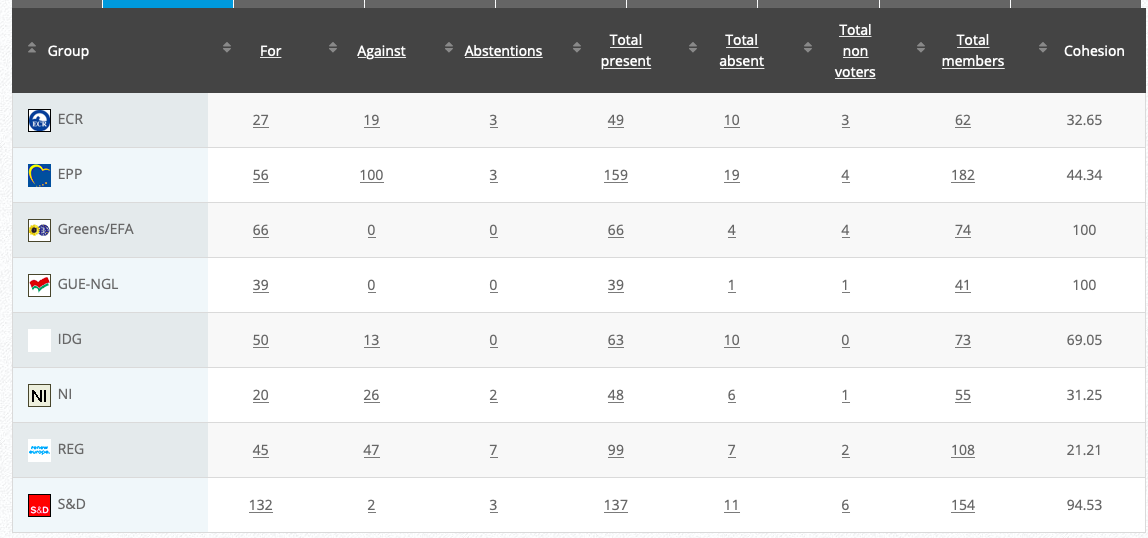
See debate above
Commission’s follow up:
6.
Objection on the assessment of the impact of plant protection products on honeybees
RPS
Co-Rapporteurs: Eric Andrieu (S&D), Martin Hojsík (Renew), Bas Eickhout (Verts/ALE), Anja Hazekamp (GUE/NGL)
Debate in Committee: 21 October 2019
Vote: For 62, Against 4, Abstentions 7 Link
Vote in Plenary: 23 October 2019
Vote: For 533, Against 67, Abstentions 100
Votewatch link
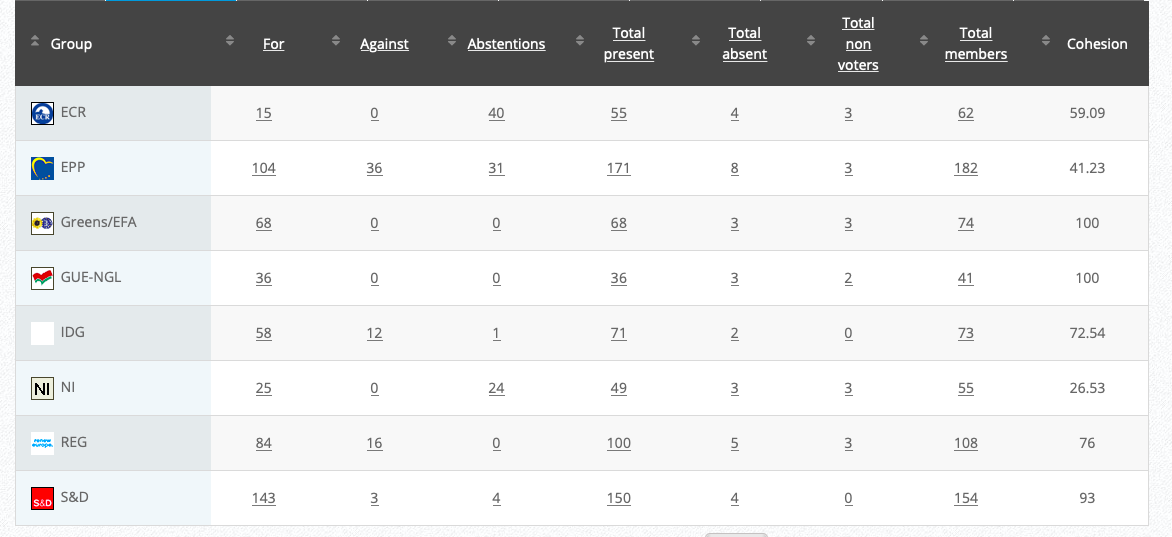
Commission’s follow up:
Objection to the authorization for a use of chromium trioxide – Cromomed
Implementing Act
Co-Rapporteurs: Maria Arena (S&D), Martin Hojsík (Renew), Bas Eickhout (Verts/ALE)
Debate in Committee: 21 October
Vote: For 43, Against 28, Abstentions 1 Link
Vote in Plenary: 24 October 2019
Vote: For 301, Against 295, Abstentions 45
Votewatch link
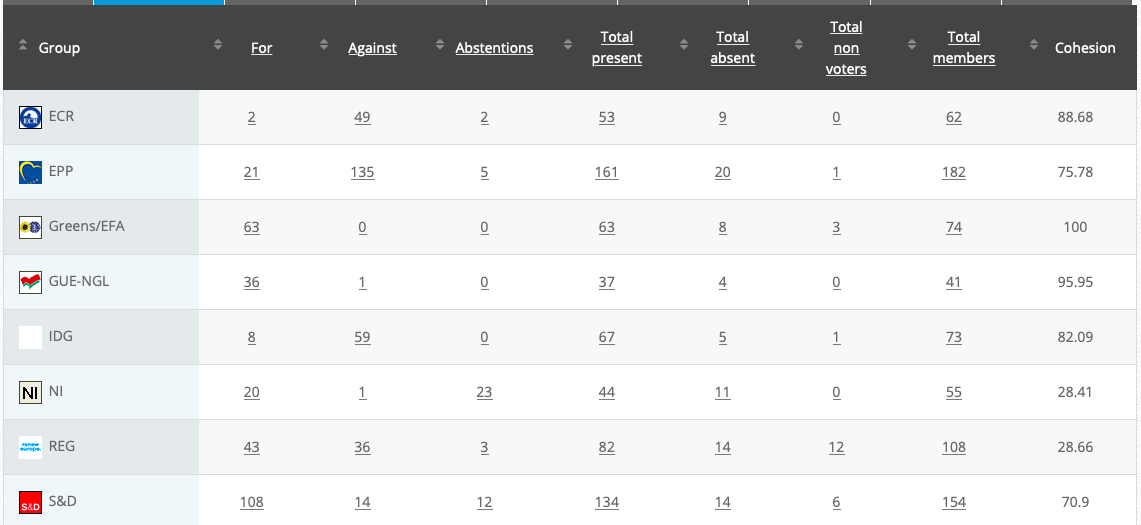
Commission’s follow up:
Objection pursuant to authorization GMO cotton LLCotton25
Implementing Act
Co-Rapporteurs: Günther Sidl (S&D), Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen
Debate in Committee: 6 November 2019
Vote: For 46, Against 25, Abstentions: 0 Link
Vote in Plenary: 14 November 2018
Vote: For 448, Against 189, Abstentions 28
Votewatch link
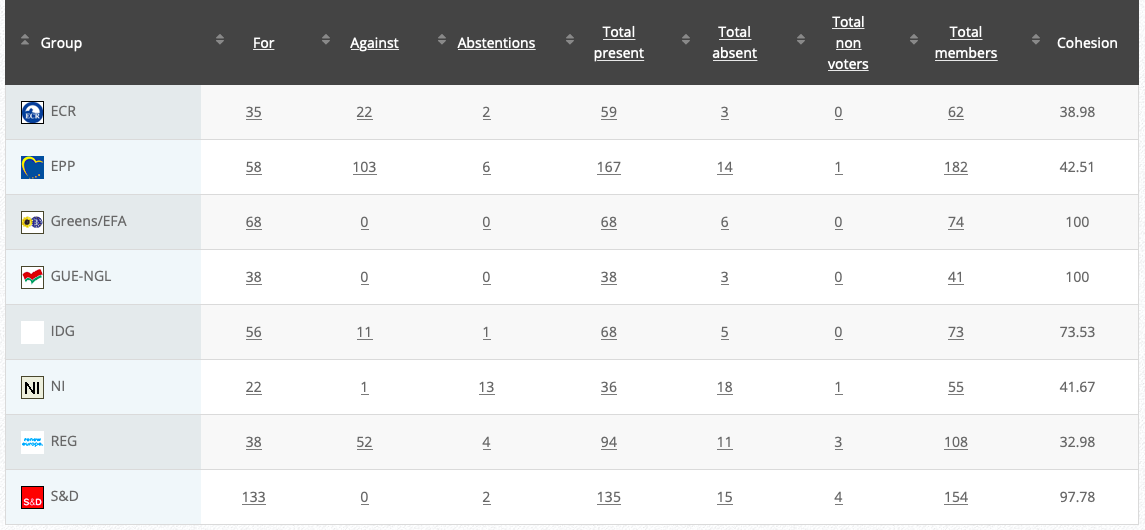
Commission’s follow up:
9.
Implementing Act
Co-Rapporteurs: Günther Sidl (S&D), Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen
Debate in Committee: 6 November 2019
Vote: For 47, Against 25, Abstention 0 Link
Vote in Plenary: 14 November 2019
Vote: For 448, Against 186, Abstentions 30
Votewatch link
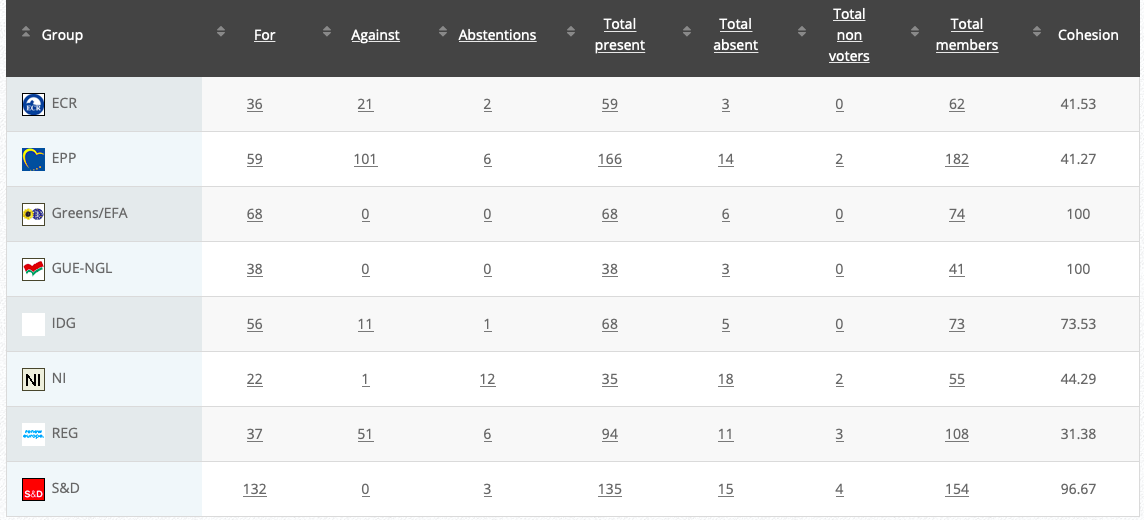
Commission’s follow up:
10.
Objection pursuant to the authorization of GMO maize MON 89034
Implementing Act
Co-Rapporteurs: Sirpa Pietikäinen, Günther Sidl (S&D),Nicolae Ştefănuță (Renew) Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI)
Debate in Committee: 6 November 2019
Vote: For 46, Against 24, Abstention 0 Link
Vote in Plenary: 14 November 2019
Vote: Vote: For 465, Against 169, Abstentions 30
Votewatch link
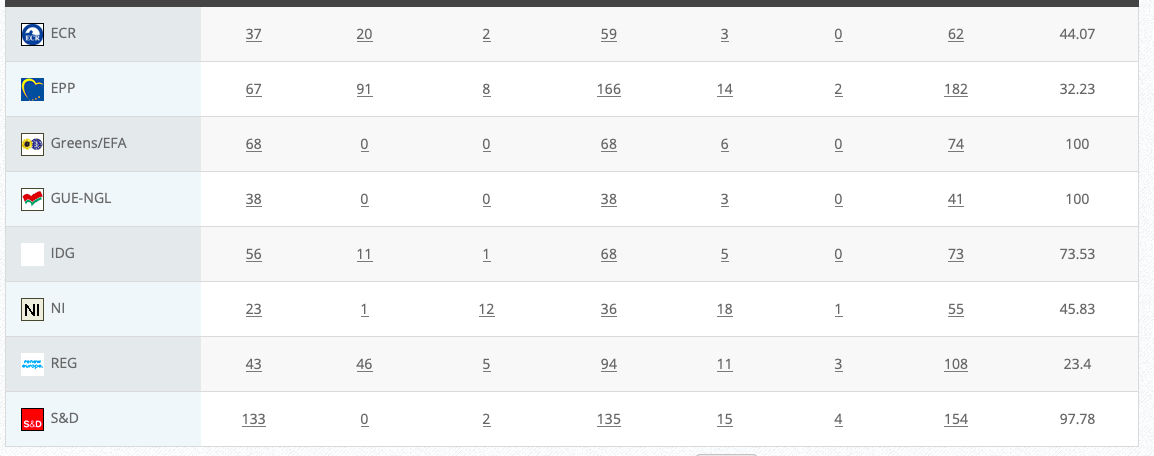
Commission’s follow up:
11.
Objection to the authorization of GMO maize Bt11
Implementing Act
Co-Rapporteurs: Günther Sidl (S&D), Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen
Debate in Committee: 6 November 2019
Vote: For 51, Against 21, Abstention 0 Link
Vote in Plenary: 14 November
Vote: For 467, Against 171, Abstentions 27
Votewatch link
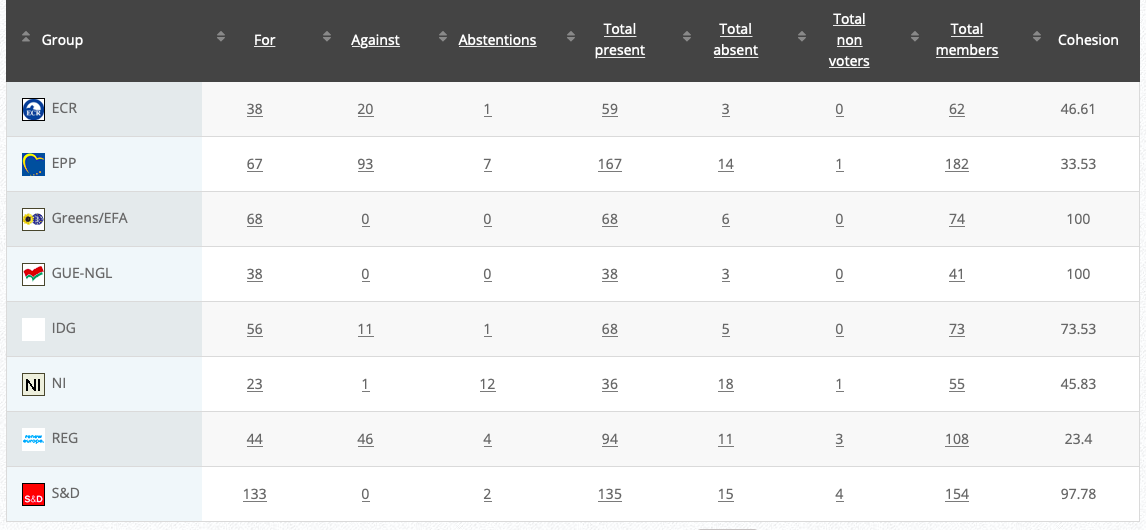
Commission’s follow up:
12.
Delegated Act
Rapporteur: Anna Zalewska (ECR)
Debate in Committee: 2 December 2019
Vote: For 19, Against 47, Abstentions 4 Link
13.
Objection to Imports of Pet Food from Saudi-Arabia
Implementing Act
Rapporteur: Joëlle Mélin (ID)
Debate in Committee: 2 December 2019
Vote: For 12, Against 58, Abstentions 1 Link
14.
Objection to the import of food from Japan
Implementing Act
Rapporteurs: Michèle Rivasi (Verts/ALE) Sirpa Pietikäinen
Debate in Committee: 2 December 2019
Vote: For 30, Against 40, Abstentions 1 Link
15.
Objection to the extension of the active substances benfluralin and others
Implementing Act
Rapporteurs: Tilly Metz (Verts/ALE), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI
Debate in Committee: 2 December 2019
Vote: For 44, Against 27, Abstentions 0 Link
Vote in Plenary: 18 December 2019
Vote: For 443, Against 216, Abstentions 33
Votewatch link
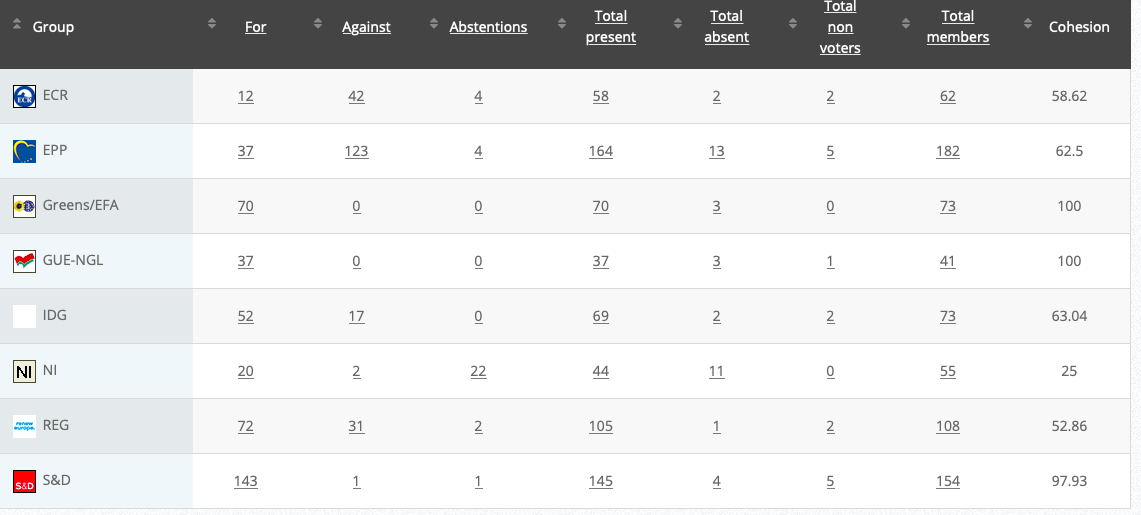
Commission’s follow up:
16.
Objection to REACH Restriction lead in PVC
RPS
Co- Rapporteurs: Bas Eickhout (Verts/ALE) Maria Arena (S&D) Martin Hojsík (Renew)
Debate in Committee: 21 January 2020
Vote: For 42, Against 22, Abstentions 4 Link
Vote in Plenary: 12 February 2020
Vote: For 394, Against 241, Abstentions 13
Votewatch link (note not on the Resolution as a whole – not listed)
Commission’s follow up:
17.
Objection to the non-approval of propolis extract
Implementing Act
Rapporteur: Joëlle Mélin (ID)
Debate in Committee: 3 February 2020
Vote: For 7, Against 59, Abstentions 5 Link
18.
Objection to authorizing GMO soybean 87708
Implementing Act
Co-Rapporteurs: Tilly Metz (Verts/ALE), Günther Sidl (S&D)Anja Hazekamp (GUE/NGL), Sirpa Pietikäinen (PPE),Eleonora Evi (NI)
Debate in Committee: 3 February 2020
Vote: For 48, Against 22, Abstentions 0 Link
Vote in Plenary: 13 May 2020
Vote: For 477, Against 181, Abstentions 23
Votewatch link
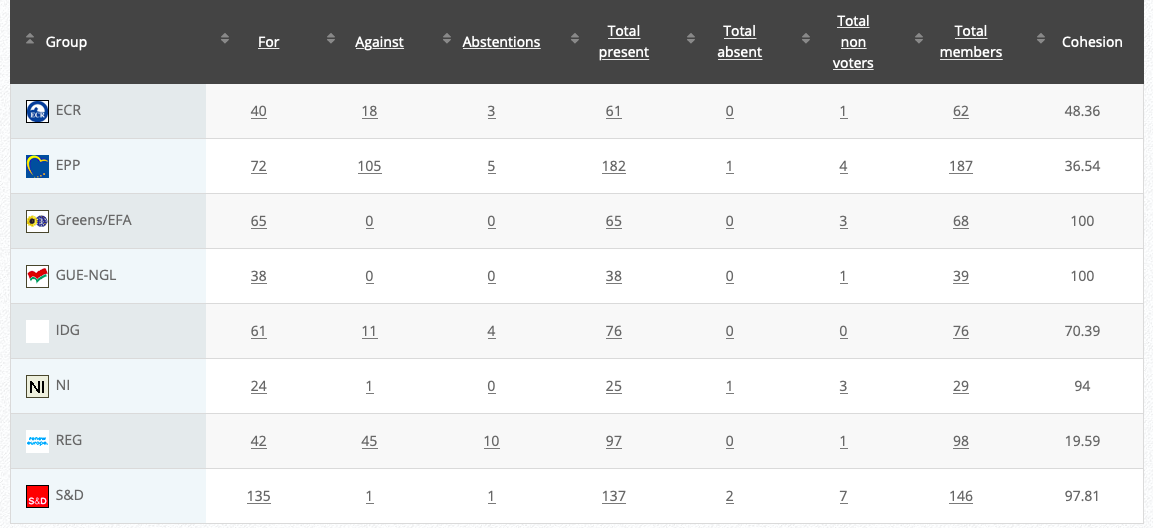
19.
Objection to maximum residue levels for cycloxydim and others
RPS
Rapporteur: Michèle Rivasi (Greens/EFA)
Debate in Committee: 21 April 2020
Vote: For 45 Against 32, Abstentions 4 Link
Vote in Plenary: 17 September 2020
Vote: For 372, Against 275, Abstentions 39
Vote watch link
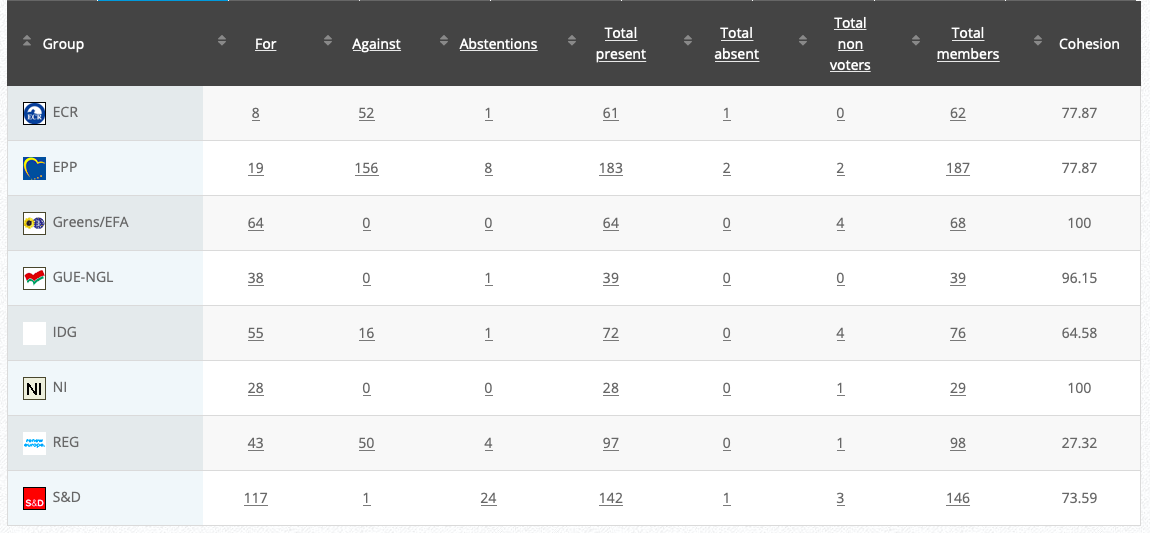
20.
Objection to renewing the approval of the active substance pyriproxyfen
Implementing Act
Rapporteur: Joëlle Mélin (ID)
Debate in Committee: 28 May 2020
Vote: For 12, Against 51, Abstentions 11 Link
21.
Objection to authorisation to REACHLaw Ltd for certain uses of chromium trioxide
Implementing Act
Co-Rapporteurs: Bas Eickhout (Verts/ALE) Maria Arena (S&D) Martin Hojsík (Renew)
Debate in Committee: 8 June 2020
Vote: For 38, Against 35, Abstentions 3 Link
Vote in Plenary: 10 July 2020
Vote: For: 325, Against 325, Abstentions 35
Votewatch link
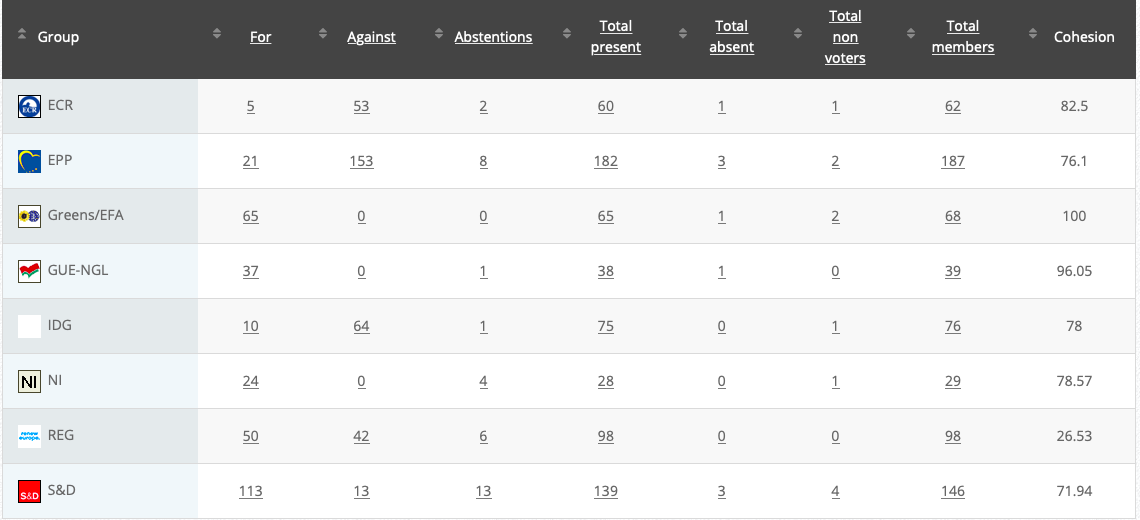
22.
Objection to the extension of the approval periods of the active substances beflubutamid and others
Implementing Act
Co-Rapporteurs: Anja Hazekamp (GUE/NGL), Maria Arena (S&D), Tilly Metz (Verts/ALE), Eleonora Evi (NI)
Debate in Committee: 8 June 2020
Vote: For 43, Against 30, Abstentions 3 Link
Vote in Plenary: 10 July 2020
Vote: For: 415, Against 252, Abstentions 20
Votewatch link
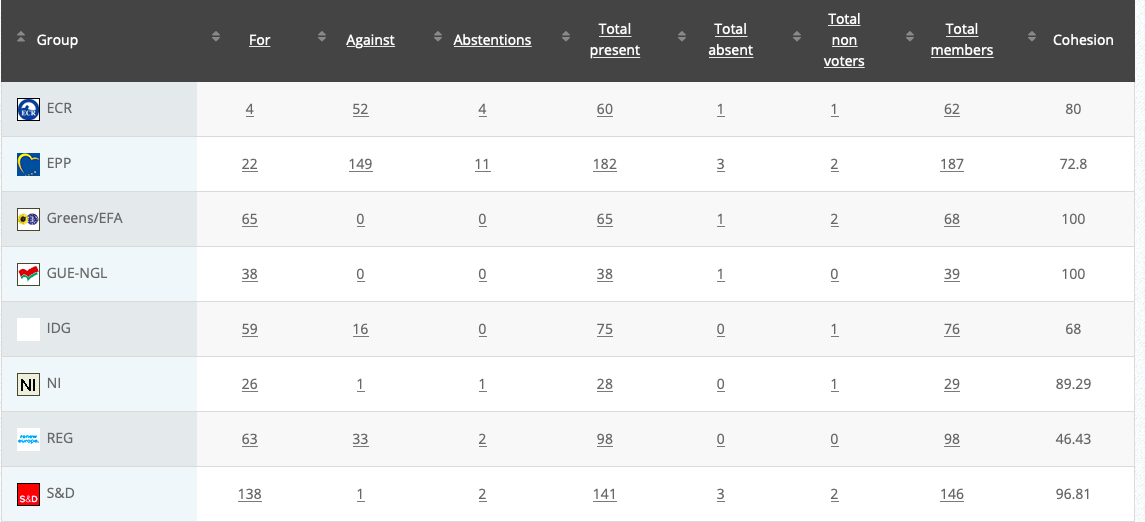
23.
Objection for food additives specifications for titanium dioxide (E 171)
RPS
Co-Rapporteurs: Michèle Rivasi, Eric Andrieu, Eleonora Evi, Joëlle Mélin, Ljudmila Novak, Mick Wallace
Debate in Committee: 7 September 2020
Vote: For 51, Against 11, Abstentions 16 Link
Vote in Plenary: 7 October 2020
Vote: For: 443, Against 118, Abstentions 135
Votewatch link
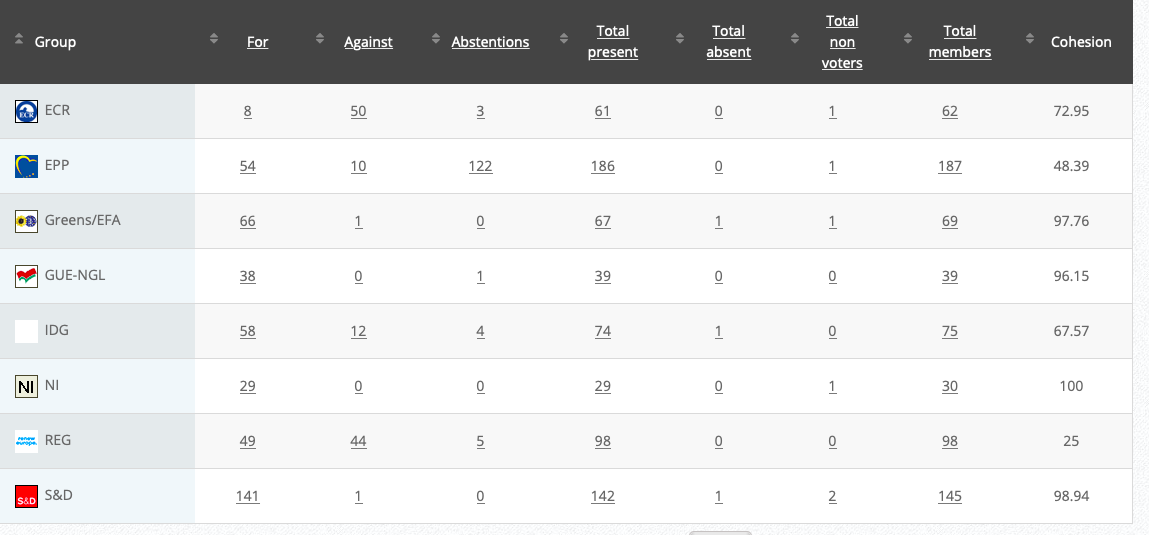
24
Objection to maximum levels of acrylamide in certain foodstuffs for infants and young children
RPS
Co-Rapporteurs: Jutta Paulus, Christel Schaldemose, Martin Hojsík, Eleonora Evi, Sirpa Pietikäinen, Mick Wallace
Debate in Committee: 28 September 2020
Vote: For 51, Against 11, Abstentions 16 Link
Vote in Plenary: 7 October 2020
Vote: For: 469, Against 137, Abstentions 90
Votewatch link
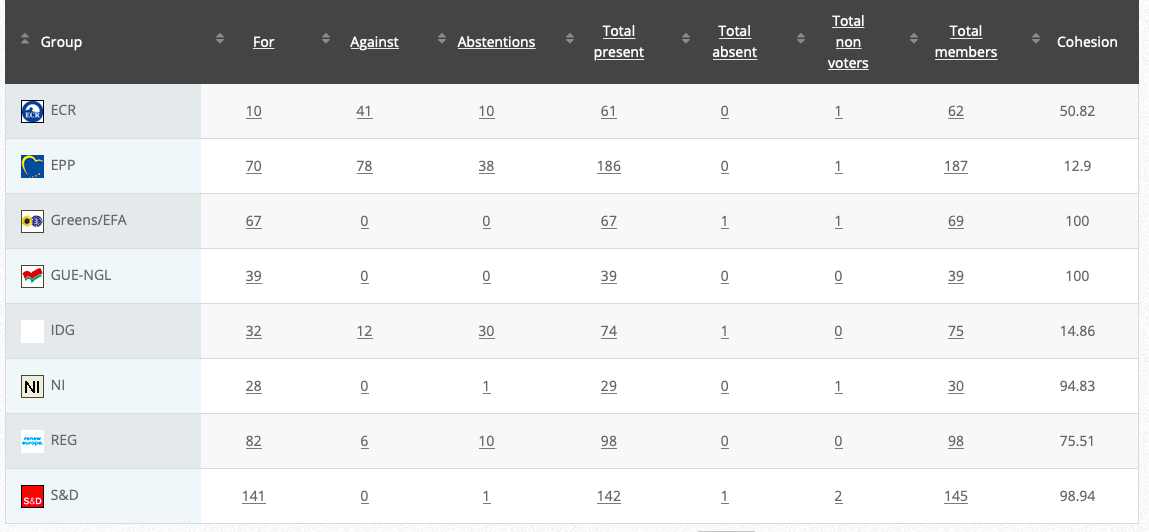
25.
Objection to Restriction on lead shot
RPS
Co-Rapporteurs: Alexander Bernhuber, Ondřej Knotek, Andrey Slabakov
Debate in Committee: 29 October 2020
Vote: For 33, Against 42, Abstentions 4 Link
Vote in Plenary: 24 November 2020
Vote: For: 292, Against 362, Abstentions 39
Votewatch link
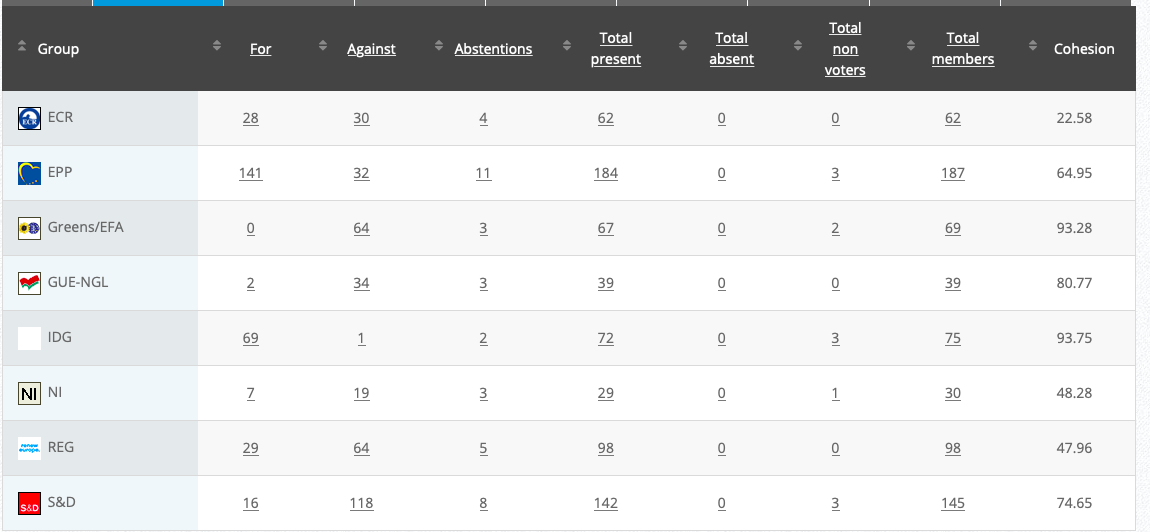
26.
Implementing Act
Co-Rapporteurs: Tilly Metz, Günther Sidl, Anja Hazekamp, Eleonora Evi, Sirpa Pietikäinen
Debate in Committee: 29 October 2020
Vote: For 51, Against 26, Abstentions 3 Link
Vote in Plenary: 11 November 2020
Vote: For: 476, Against 178, Abstentions 25
Votewatch link
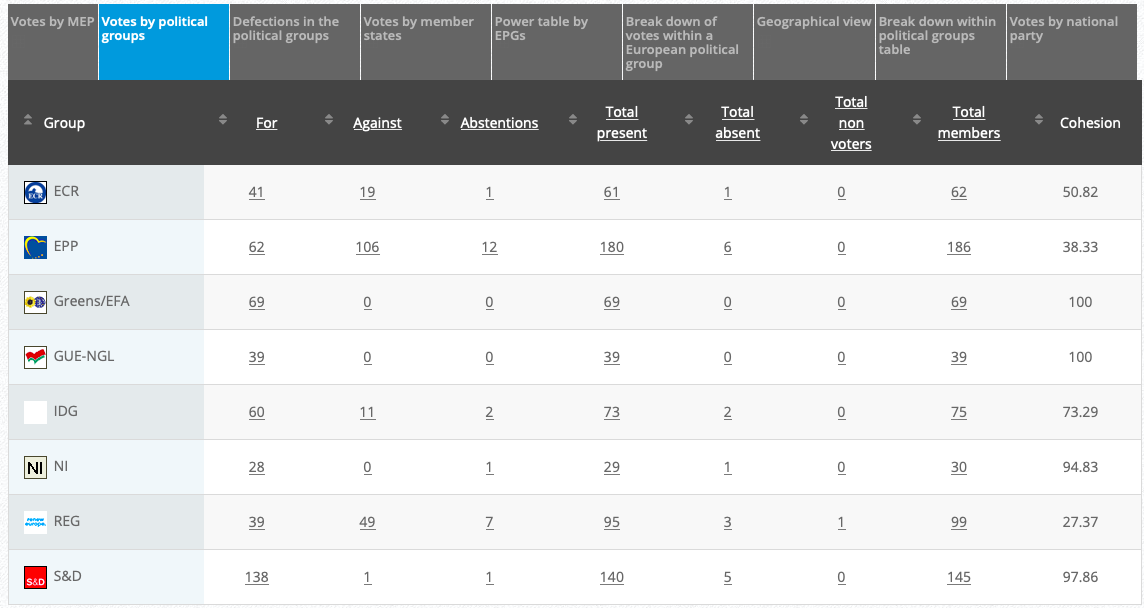
27.
Objection to GMO maize MON 87427
Implementing Act
Co-Rapporteurs: Tilly Metz, Günther Sidl, Anja Hazekamp, Eleonora Evi, Sirpa Pietikäinen
Debate in Committee: 29 October 2020
Vote: For 52, Against 25, Abstentions 3 Link
Vote in Plenary: 11 November 2020
Vote: For: 483, Against 178, Abstentions 25
Votewatch link
See debate above
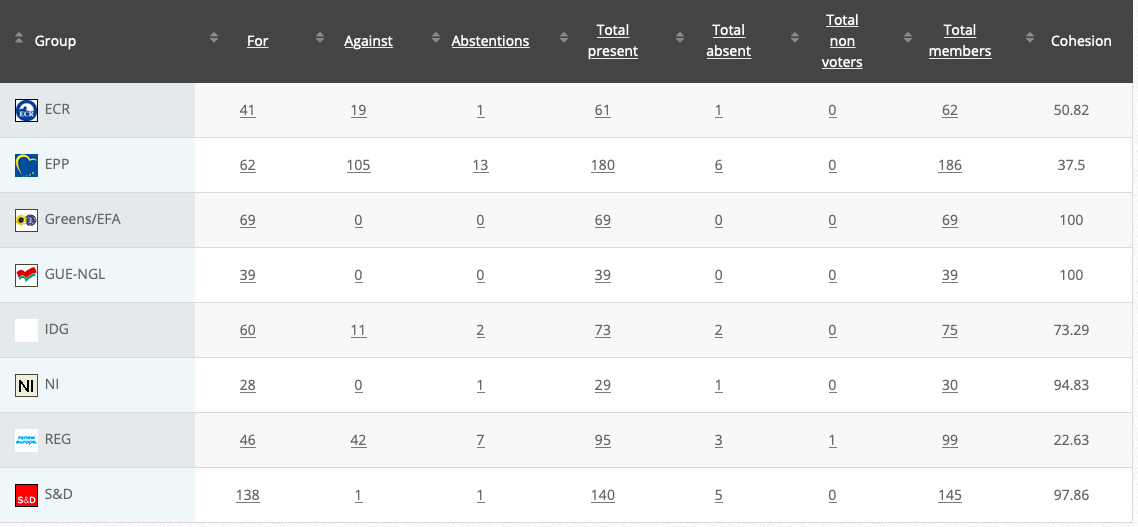
28.
Implementing Act
Co-Rapporteurs: Tilly Metz, Günther Sidl, Anja Hazekamp, Eleonora Evi, Sirpa Pietikäinen
Debate in Committee: 29 October 2020
Vote: For 57, Against 21, Abstentions 2 Link
Vote in Plenary: 11 November 2020
Vote: For 526, Against 142, Abstentions 18
Votewatch link
See debate above
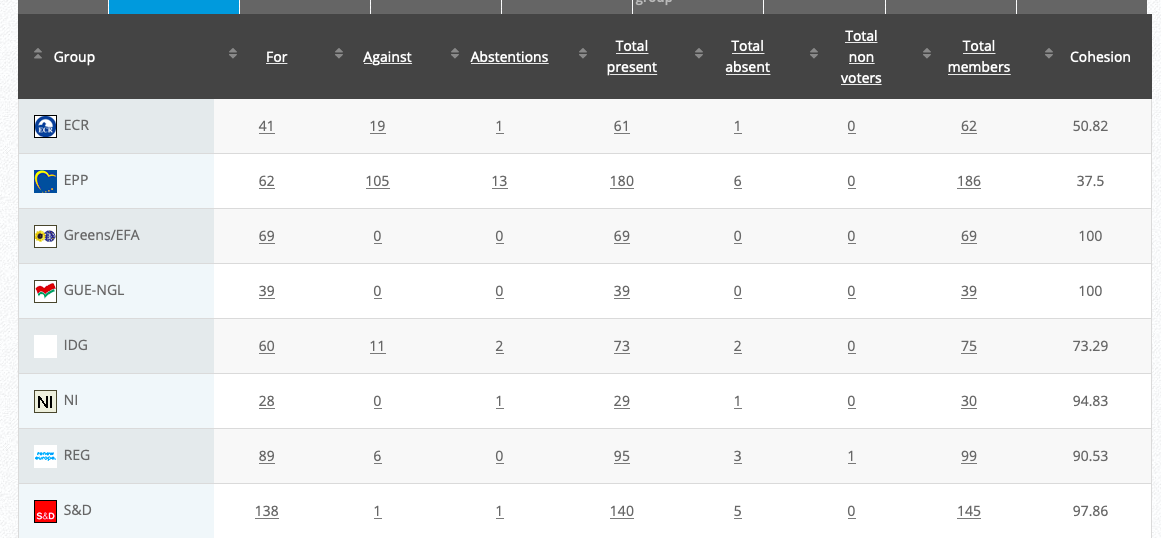
29.
Objection to approving carbendazim as an active substance in biocidal products
Implementing Act
Co-Rapporteurs: Maria Arena, Michèle Rivasi, Anja Hazekamp, Eleonora Evi
Debate in Committee: 16 November 2020
Vote: For 48, Against 26, Abstentions 3 Link
Vote in Plenary: 25 November 2020
Vote: For: 458, Against 219, Abstentions 19
Votewatch ink
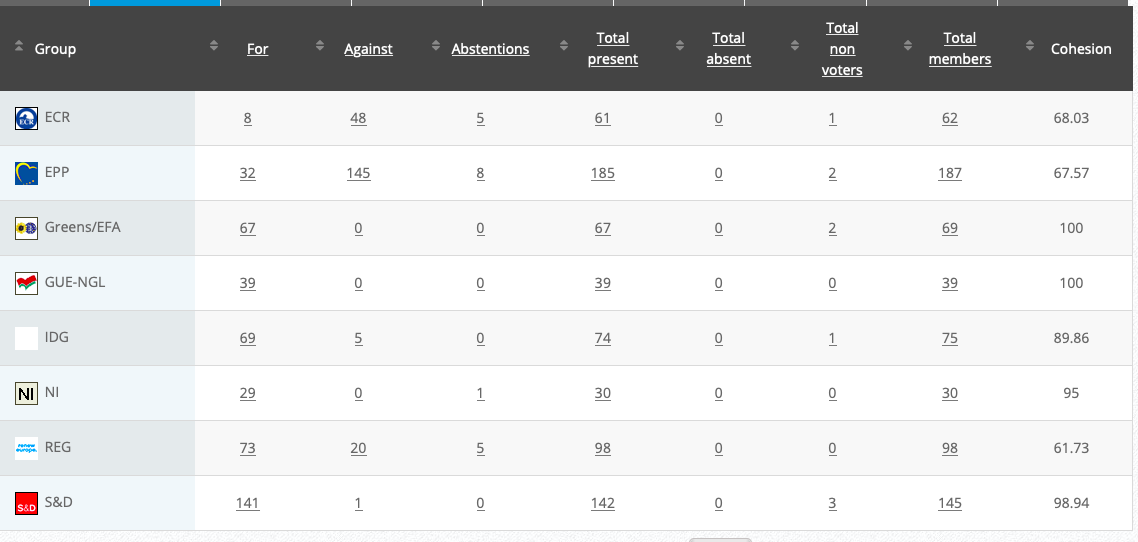
30.
Objection to the extension of the active substances chlorotoluron,and others
Implementing Act
Co-Rapporteurs: Anja Hazekamp, Maria Arena, Tilly Metz, Eleonora Evi
Debate in Committee: 16 November 2020
Vote: For 45, Against 28, Abstentions 4 Link
Vote in Plenary: 25 November 2020
Vote: For: 425, Against 231, Abstentions 40
Votewatch link
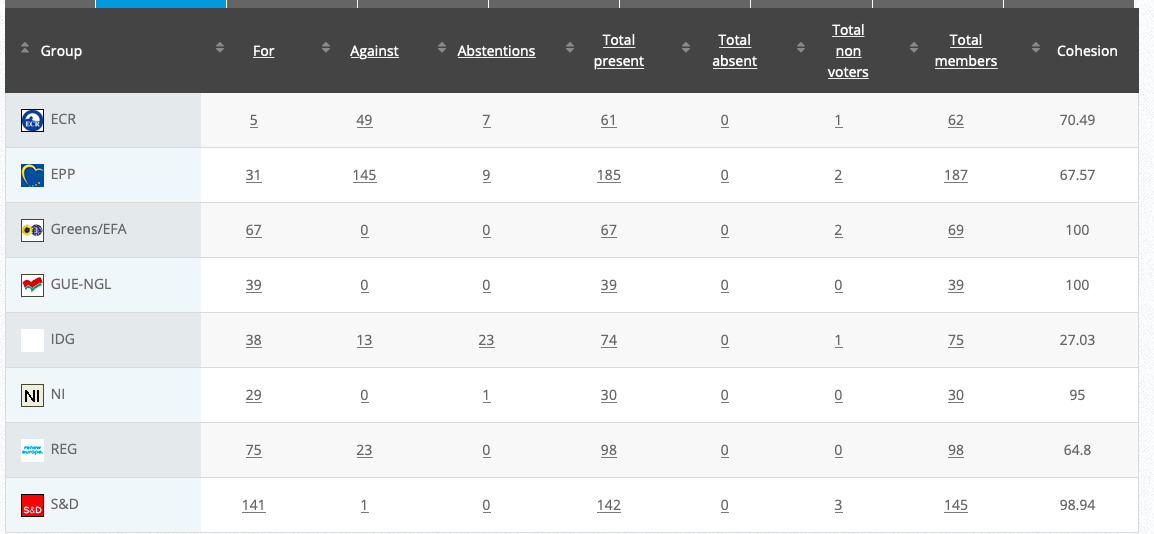
31.
Objection to GMO soy bean MON 87751
Implementing Act
Co-Rapporteurs: Tilly Metz (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen (PPE)
Vote in Committee:30 November 2020
Vote: For 50, Against 27, Abstentions 3 Link
Vote in Plenary: 17 December 2020
Vote: For: 472, Against 194, Abstentions 30
Votewatch link
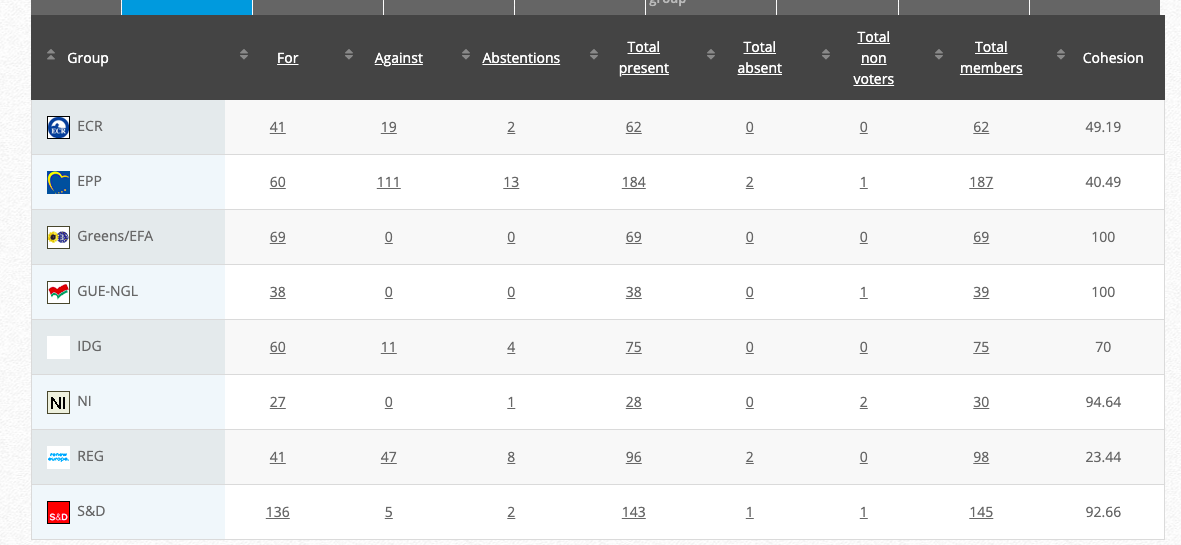
32.
Objection to genetically modified maize 87427
Implementing Act
Co-Rapporteurs: Tilly Metz (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen (PPE)
Debate in Committee:30 November 2020
Vote: For 53, Against 21, Abstentions 1 Link
Vote in Plenary: 17 December 2020
Vote: For: 488, Against 186, Abstentions 22
Votewatch link
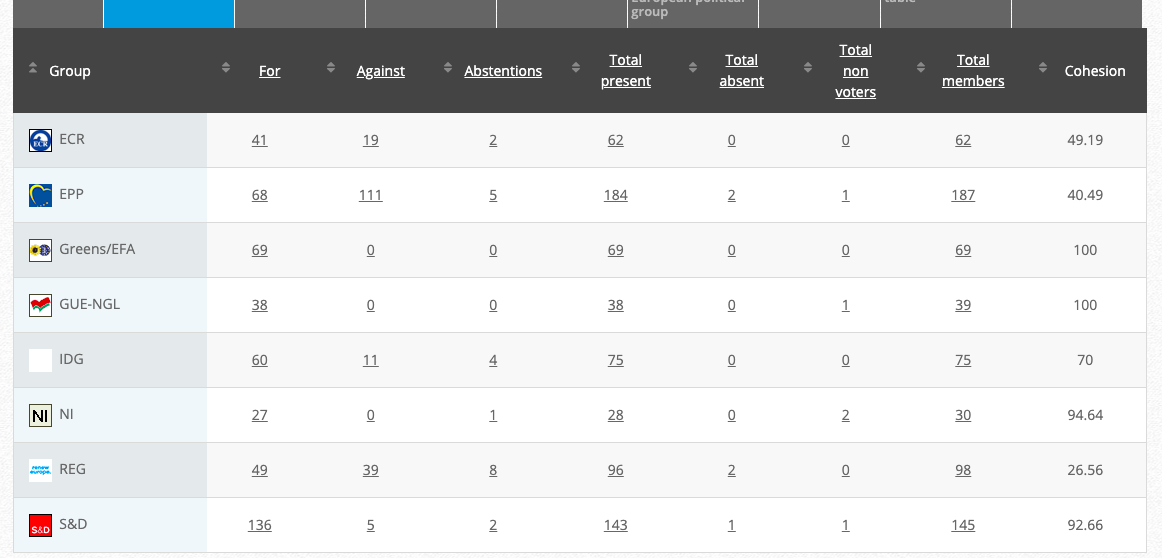
33.
Objection to genetically modified maize MON 89034
Implementing Act
Co-Rapporteurs: Tilly Metz (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen (PPE)
Debate in Committee: 30 November 2020
Vote: For 53, Against 26, Abstentions 1 Link
Vote in Plenary:17 December 2020
Vote: for 488, against 186, abstentions: 22
Votewatch link
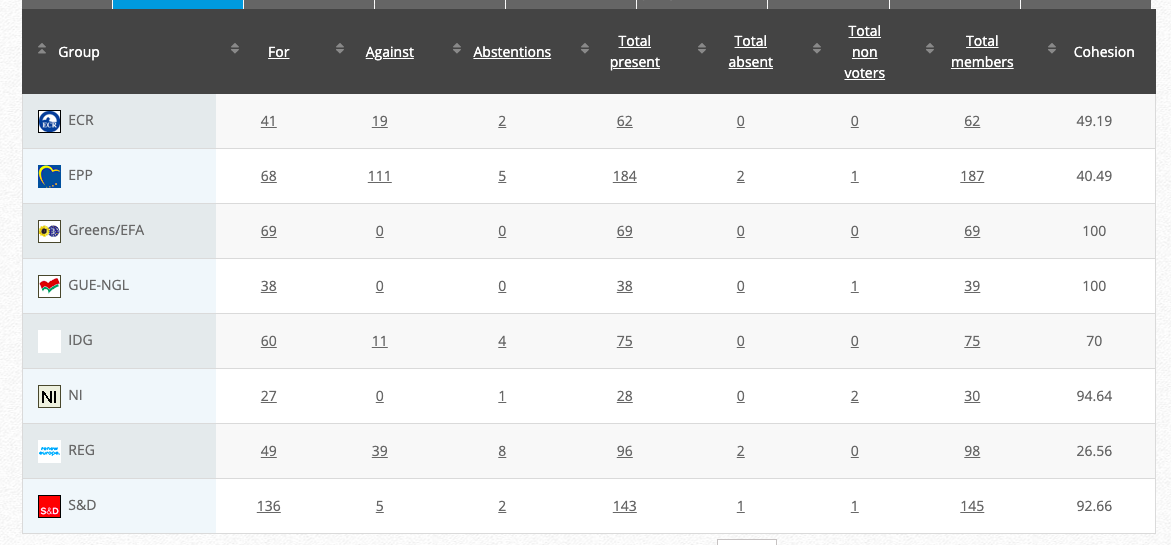
34.
Objection to genetically modified maize MIR604 (renewal)
Implementing Act
Co-Rapporteurs: Tilly Metz (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen (PPE)
Debate in Committee: 30 November 2020
Vote: For 53, Against 26, Abstentions 1 Link
Vote in Plenary: 17 December 2020
For: 489, Against 185, Abstensions: 22
Votewatch link
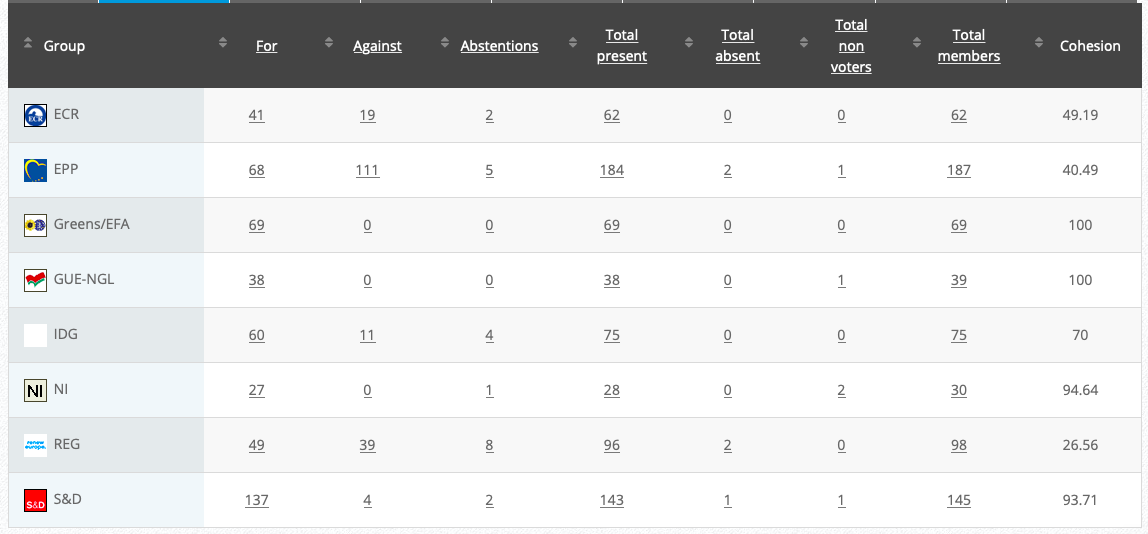
35.
Genetically modified soybean MON 87751 × MON 87701 × MON 87708 × MON 89788
Implementing Act
Co-Rapporteurs: Tilly Metz (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen (PPE)
Debate in Committee: 30 November 2020
Vote: For 50, Against 27, Abstentions 3
Vote in Plenary: 17 December 2020
Vote: 472, Against 194, Abstentions 30
Votewatch link
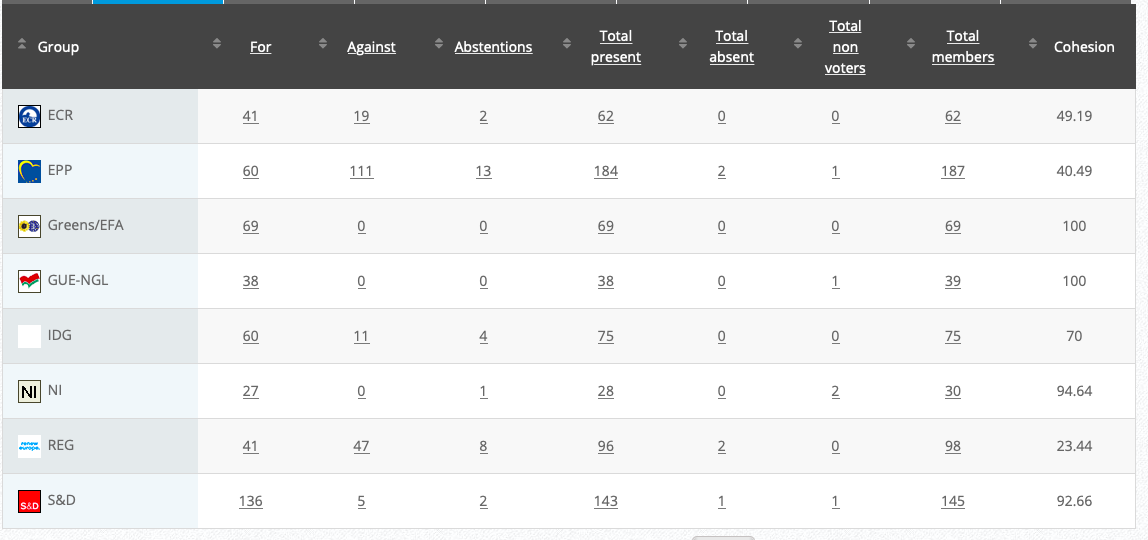
36.
Implementing Act
Co-Rapporteurs: Tilly Metz (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (GUE/NGL), Eleonora Evi (NI), Sirpa Pietikäinen (PPE)
Debate in Committee: 30 November 2020
Vote: For 50, Against 27, Abstentions 3 Link
Vote in Plenary: 17 December 2020
Vote: 472, Against 194, Abstentions 30
37.
30 November 2020 –
Implementing Act
Rapporteur: Pascal Canfin
Link Minutes
Vote: For 75, Against 1, Abstentions 4
38.
Objection to MRLs for aclonifen and others
RPS
Rapporteur: Joëlle Mélin
Debate in Committee: 10 December 2020
Vote: For 9, Against 57, Abstentions 13 Link
39.
Objection to MRLs for carbon tetrachloride
RPS
Rapporteur: Joëlle Mélin
Debate in Committee: 10 December 2020
Vote: For 10, Against 65, Abstentions 5 Link
See debate above
40.
Objection to MRLs for fluxapyroxad
RPS
Rapporteur: Joëlle Mélin
Debate in Committee: 10 December 2020
Vote: For 9, Against 70, Abstentions 0 Link
42.
Objection to non-approval of cayenne extract
Implementing Act
Rapporteur: Joëlle Mélin
Debate in Committee: 26 January 2021
Vote: For 9, Against 70, Abstentions 0 Link
43
44
Implementing Act
Rapporteurs: Anja Hazekamp, Maria Arena, Tilly Metz
Vote in Committee: 24 February 2021
Vote: For 49, Against 29, Abstention 1 Link
Vote in Plenary: 10 March 2021
Vote for 472, Against 214, Abstentions 9
Voewatch link
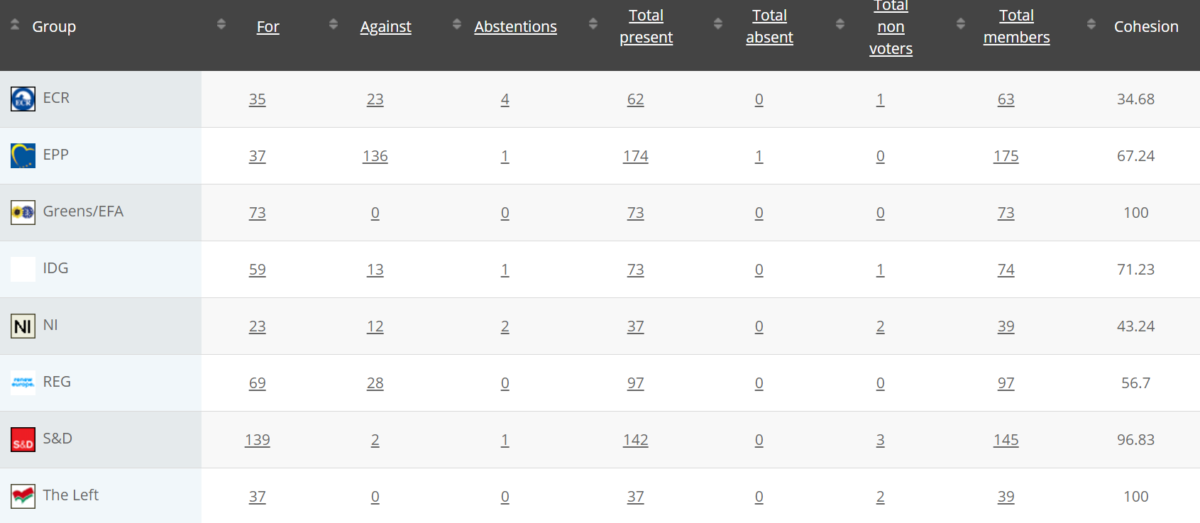
45
Implementing Act
Rapporteurs: Martin Häusling, Günther Sidl, Anja Hazekamp, Sirpa Pietikäinen
Vote in Committee: 25 February 2021
Votes: For 53, Against 25, Abstention 1 Link
Vote in Plenary: 10 March 2021
Votes: For 491, Against 184, Abstentions 20
Votewatch link
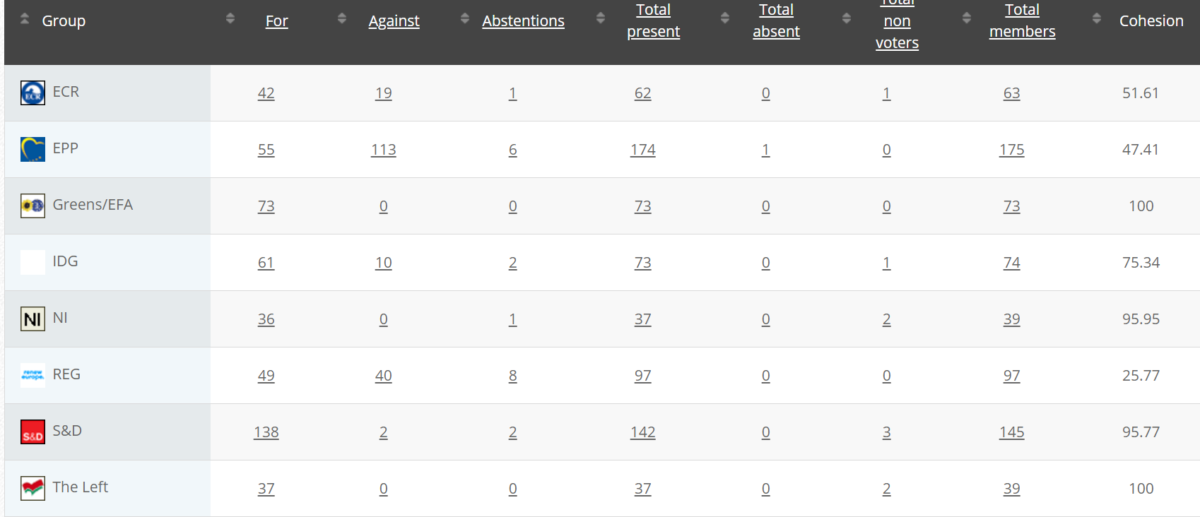
46
Implementing Act
Rapporteurs:Martin Häusling, Günther Sidl, Anja Hazekamp, Sirpa Pietikäinen
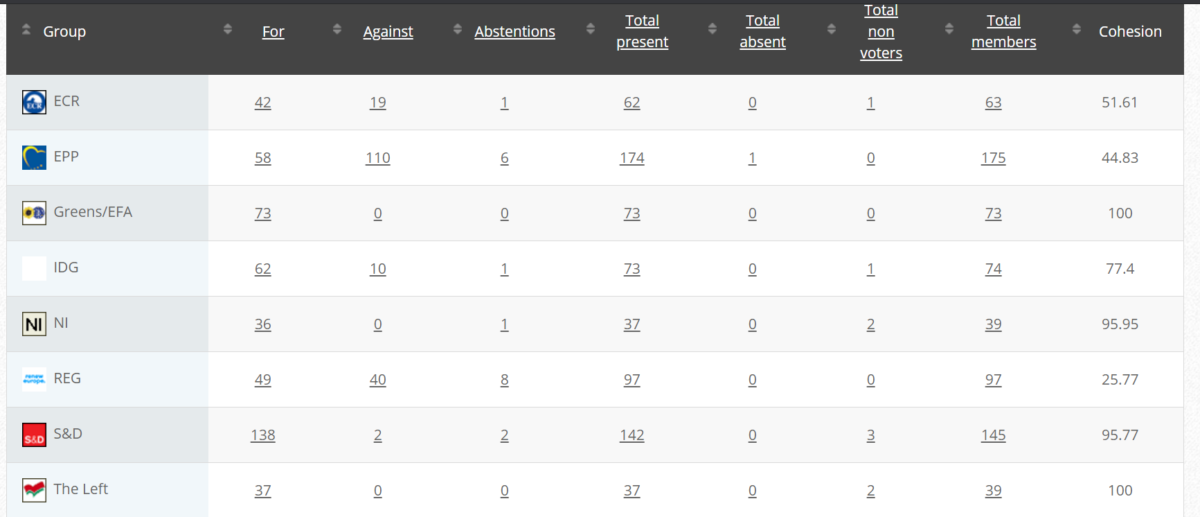
49. Objection to draft Commission Implementing Regulation amending Regulation (EU) No 37-2010 to classify the substance imidacloprid as regards its maximum residue limit in foodstuffs of animal origin
RPS Measure
Objectors: Grace O’Sullivan (Greens/EFA)
Vote in Committee: 27 May 2021
Vote: For 49, Against 27, Abstain 2
Plenary 9 June 2021
Vote: For 441, Against 232, Abstain 18
50.Objection to Commission Implementing Regulation (EU) amending Implementing Regulation(EU) 540-2011as regards the extension of the approval periods ofseveral active substances, including flumioxazine
Implementing Act
Objectors: AnjaHazekamp (TheLeft),MariaArena(S&D), Tilly Metz (Greens/EFA)
Vote in Committee: 27 May 2021
Vote: For 49, Against 27, Abstain 2
Plenary 9 June 2021
Vote: 434, Against 230, Abstain 27
51. Objection pursuant to Rule 112(2) and (3): authorising the placing on the market of products containing, consisting of or produced from genetically modified soybean DAS- 81419-2 pursuant to Regulation(EC) No 1829/2003ofthe European Parliament and of the Council –
Co-rapporteurs: Martin Häusling (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (The Left), Sirpa Pietikäinen (PPE))
Implementing act
Vote: 21 June
Vote: For 48, Against 28, Abstentions 3
Vote in Plenary: 6 July 2021
Vote For 470, Against 199, Abstentions 23
52. Objection pursuant to Rule 112(2) and (3): authorising the placing on the market of products containing, consisting of or produced from genetically modified soybean DAS- 81419-2 × DAS–44406–6, pursuant to Regulation (EC) No 1829/2003 of the European
Co-rapporteurs: Martin Häusling (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (The Left), Sirpa Pietikäinen (PPE)
Implementing act
Vote: 21 June 2021
Vote: For 49, Against 27, Abstentions 3
Vote in Plenary: 6 July 2021
Vote For 470, Against 199, Abstentions 23
53. Objection pursuant to Rule 112(2) and (3): authorising the placing on the market of products containing, consisting of or produced from genetically modified maize 1507 × MIR162 × MON810 × NK603 and genetically modified maize combining two or three of the single events 1507, MIR162, MON810 and NK603, pursuant to Regulation (EC) No 1829/2003 of the European Parliament and of the Council – 1234186ENv2
Co-rapporteurs: Martin Häusling (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (The Left), Sirpa Pietikäinen (PPE)
Implementing act
Vote: 21 June 2021
Vote: For 49, Against 27, Abstentions 3
Vote in Plenary: 6 July 2021
Vote For 470, Against 200, Abstentions 22
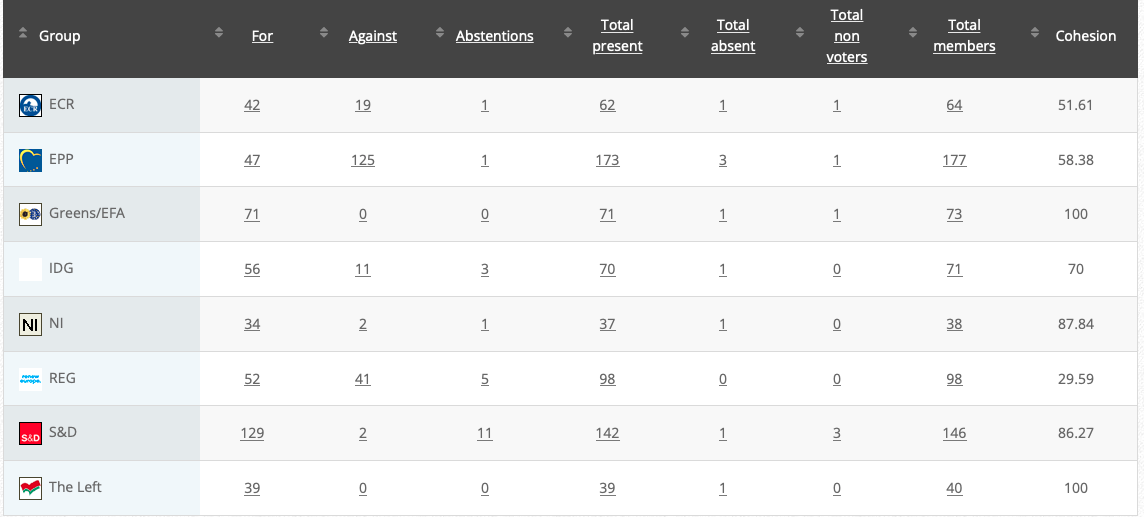
54. Objection pursuant to Rule 112(2) and (3): renewing the authorisation for the placing on the market of products containing, consisting of or produced from genetically modified maize Bt 11 (SYN-BTØ11-1) pursuant to Regulation (EC) No 1829/2003 of the European Parliament and of the Council –1234187Bt11
Co-rapporteurs: Martin Häusling (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (The Left), Sirpa Pietikäinen (PPE)
Implementing act
Vote: 21 June 2021
Vote: For 49, Against 28, Abstentions 2
Vote in Plenary: 6 July 2021
Vote For 470, Against 200, Abstentions 22
55. Commission Regulation amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards the prohibition to feed non-ruminant farmed animals, other than fur animals, with protein derived from animals (RE_Objection_RPS_animals_proteins_EN)
Co- rapporteurs: Piernicola Pedicini (Verts/ALE), Anja Hazekamp (The Left))
Implementing act
Vote: 21 June 2021
Vote: For 35, Against 39, Abstentions 5
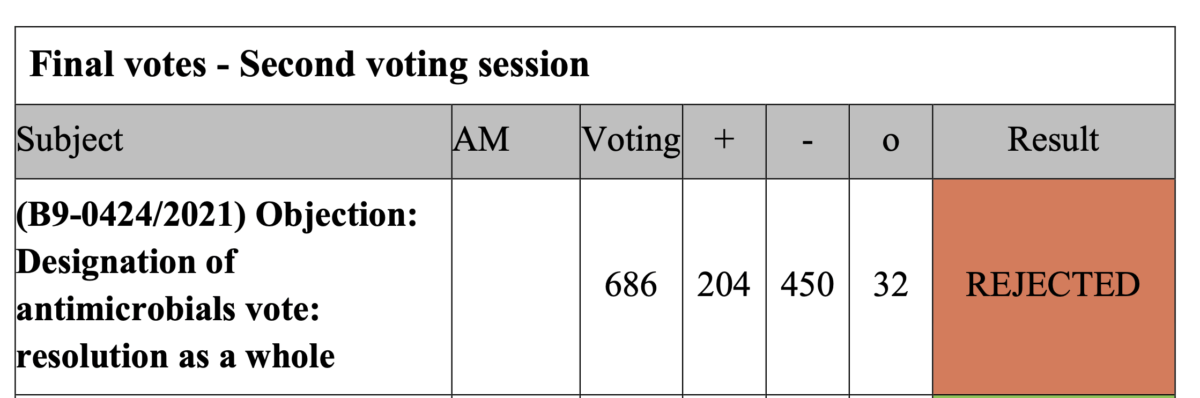
56. Objection on the Commission delegated regulation of 26 May 2021 supplementing Regulation (EU) 2019/6 of the European Parliament and of the Council by establishing the criteria for the designation of antimicrobials to be reserved for the treatment of certain infections in humans (link)
Rapporteurs: Martin Häusling (Verts/ALE)
Delegated act
Vote: 12 July 2021
Vote: For 38, Against 18, Abstentions 22
Vote in Plenary: 15 September July 2021
Vote For 204, Against 450, Abstentions 32
57. Objection pursuant to Rule 112(2) and (3) to Implementing Regulation (EU) No 540/2011 as regards the extension of the approval periods of the active substances, including chlorotoluron and difenoconazole (link)
Rapporteurs: Anja Hazekamp, Maria Arena, Tilly Metz
Implementing act
Date: 27 September 2021
Vote: For 47, Against 30, Abstentions 0
Vote in Plenary:5 October 2021
Vote For 407, Against 256, Abstentions 24
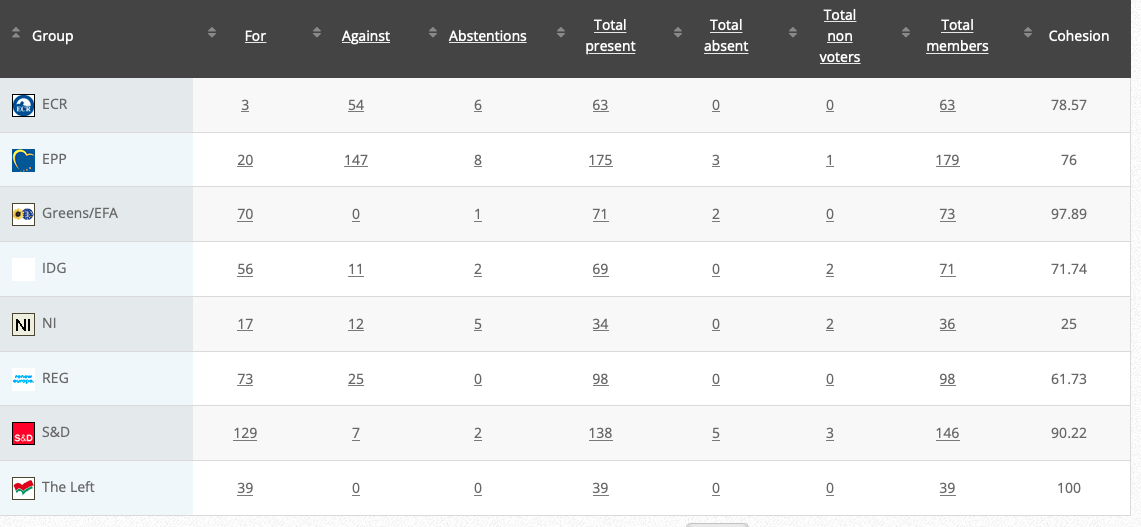
58. Commission Delegated Regulation supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by establishing the technical screening criteria for determining the conditions under which an economic activity qualifies as contributing substantially to climate change mitigation or climate change adaptation and for determining whether that economic activity causes no significant harm to any of the other environmental objectives- Joint ECON-ENVI (CJ36) (link)
Rapporteurs: Nicola Beer, Jessica Polfjärd, Andreas Glück, Emma Wiesner
Delegated act
Vote: 27 September 2021
Vote: For 34, Against 92, Abstentions 4
Vote in Plenary: 5 October 2021
Vote: For 227, Against 428, Abstentions 31
59. Objection pursuant to Rule 112(2) and (3) and (4)(c) of the Rules of Procedure on the draft Commission regulation amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for flonicamid in or on certain products (link)
Rapporteurs: Michèle Rivasi (Greens/EFA
Implementing act
Vote: 6 December 2021
Vote: For 16, Against 53, Abstentions 5 (Link)
Rapporteurs: Martin Häusling (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (The Left), Sirpa Pietikäinen (EPP)
Implementing act
Vote: 13 January 2022
Vote: For 45, Against 31, Abstentions 2 (link)
Vote in Plenary: 15 February 2022
Vote: For 475, Against 209, Abstenstions 15
Rapporteurs: Martin Häusling (Verts/ALE), Günther Sidl (S&D), Anja Hazekamp (The Left), Sirpa Pietikäinen (PPE)
Implementing act
Vote: 13 January 2022
Vote: For 45, Against 31, Abstentions 2 (link)
Vote in Plenary: 15 February 2022
Vote: For 477, Against 207, Abstentions 15
Rapporteurs: Sylvia Limmer (ID)
Implementing act
Vote: 10 February 2022
Vote: For 9, Against 71, Abstentions 2 (link)
Co-rapporteurs: Martin Häusling (Verts/ALE), Anja Hazekamp (The Left), Sirpa Pie tikäinen (PPE), Günther Sidl (S&D)
Implementing act
Vote: 10 February 20022
Vote: For 56, Against 29, Abstentions 2 (link)
Vote in Plenary: 9 March 2022
Vote: For 428, Against 198, Abstentions 14
Co-rapporteurs: Martin Häusling (Verts/ALE), Anja Hazekamp (The Left), Sirpa Pietikäinen (PPE), Günther Sidl (S&D)
Implementing act
Vote: 10 February 20022
Vote: For 56, Against 29, Abstentions 2 (link)
Vote in Plenary 9 March 2022
Vote: For 474, Against 205, Abstentions 15
Co-rapporteurs: Jutta Paulus (Verts/ALE), Maria Arena (S&D), Sirpa Pietikäinen (PPE), Mick Wallace (The Left))
RPS
Vote: 15 March 20022
Vote: For 47, Against 38, Abstentions 1 (link)
Vote in Plenary 24 March 2022
Vote: Rejected
Co-rapporteurs: Martin Häusling (Greens/EFA), Anja Hazekamp (The Left), Sirpa Pietikäinen (EPP), Günther Sidl (S&D))
Implementing act
Vote: 31 March 2022
Vote: For 53, Against 31, Abstentions 1 (link)
Vote in Plenary: 6 April 2022
Vote: For 420, Against 189, Abstentions 16
Adoption of motion for a resolution tabled by Michèle Rivasi (Verts/ALE) In favour 23
Date: 14 June 2022
Votes: For 23, Against 56, Abstention 1
Adoption of motion for a resolution tabled by Joëlle Mélin (ID)
Implementing act
Date: 14 June 2022
Votes: For 6, Against 70, Abstentions 3
Co-Rapporteurs: Martin Häusling (Greens/EFA), Anja Hazekamp (The Left), Sirpa Pietikäinen (EPP), Günther Sidl (S&D)
Implementing act
Vote: 14 June 2022
Vote: For 53, Against 26, In favour 53, Abstention 1
Co-Rapporteurs: Martin Häusling (Greens/EFA), Anja Hazekamp (The Left), Sirpa Pietikäinen (EPP), Günther Sidl (S&D)
Implementing act
Vote: 13 June
Vote: For 52, Against 27, Abstention 1
Co-rapporteur: Martin Häusling (Greens/EFA), Tiemo Wölken (S&D), Nicolae Ştefănuță (Renew), Anja Hazekamp (The Left)
Implementing act
Vote: 13 June
Vote: For 48, Against 27, Abstention
Vote in Plenary: Date
Vote:
Joint committee procedure (Rule 58) Committee on Economic and Monetary Affairs and Committee on the Environment, Public Health and Food Safety
Co-rapporteurs: : Othmar Karas, Christophe Hansen, Alexander Bernhuber, Sirpa Pietikäinen, Paul Tang, Simona Bonafè, Martin Hojsík, Linea Søgaard-Lidell, Emma Wiesner, Monica Semedo, Claudia Gamon, Róża Thun und Hohenstein, Bas Eickhout and Michael Bloss (on behalf of the Greens/EFA), Silvia Modig and Dimitrios Papadimoulis (on behalf of the LEFT), Evelyn Regner, Rasmus Andresen, Jutta Paulus, Marie Toussaint, Roman Haider, Mick Wallace, Nikolaj Villumsen, Anja Hazekamp, Cornelia Ernst, Malin Björk, José Gusmão, Marisa Matias, Idoia Villanueva Ruiz, Martin Schirdewan, Chris MacManus, Manon Aubry, Manuel Bompard, Petros Kokkalis
Delegated act
14 June
Vote: For 76, Against 62, Abstention 4
Vote in Plenary: 6 July 2022
Votes: For 278, Against 328, Abstentions 33